Hemodynamics is the study of the dynamic behavior of blood. As blood flows from chamber to chamber,
as valves open and close, and as the myocardium contracts and relaxes, pressures are generated in various
parts of the heart. These cardiovascular pressures can be measured and monitered through catheters whose tips are placed in the atria, pulmonary artery or systemic arteries.
These are called "hemodynamic lines".
Hemodynamic lines have several uses. They enable you to sample venous and arterial blood without having to stick a patient over and over. They provide a way to monitor various waveforms, which can provide clues
to patient status. The combination of pulmonary, arterial, and systemic arterial lines can be used to calculate cardiac output.
Most important, these lines enable you to monitor directly various cardiac pressures. Interpretation of these pressures can guide you and the physician in planning and evaluating
therapy in shock, fluid overload or deficit, cardiac failure, and other conditions.

 PRESSURE MEASUREMENTS
PRESSURE MEASUREMENTS
The most important cardiac pressure is that of the left ventricle., because it is a major determinant of systemic perfusion. The pressure in the left ventricle just before systole is called the
left ventricular end-diastolic pressure or LVEDP. This pressure reflects the compliance of the left ventricle - it's ability to receive blood from the left atrium during diastole. When the left
ventricular compliance decreases, the LVEDP rises. MI and left ventricular failure are two examples of when left ventricular compliance decreases.
 CORRELATION OF PRESSURES
CORRELATION OF PRESSURES
There is a close correlation between LVEDP and other cardiac pressures. In the presence of a normal mitral valve, LVEDP is reflected by left atrial pressure or LAP. In the person with a normal mitral valve and normal lung function, the LVEDP is also reflected by the pressure in the pulmonary capillary
bed,pulmonary capillary wedge pressure or PCWP) and the pressure in the pulmonary artery at the end of diastole. This latter pressure is sometimes referred to as the pulmonary artery end diastolic pressure or PAEDP.
 Remember, this concept only hold true for patients with a normal mitral valve and no pulmonary disease.
Remember, this concept only hold true for patients with a normal mitral valve and no pulmonary disease.
Left arterial pressure can be monitored at bedside, but a LAP line can be dangerous because it provides a direct path for air or clots to enter the left ventricle and become systemic emboli. The pulmonary capillary and pulmonary arterial pressures can be monitored at the bedside with a balloon-tipped
catheter placed in the pulmonary artery. With the balloon deflated, one can measure pulmonary artery systolic, diastolic, and mean pressures with the catheter. When the balloon is inflated, it wedges the catheter in a small distal branch
of the pulmonary artery. The pressure recorded is that reflected back from the left atrium through the pulmonary capillary bed. This pressure is the
Pulmonary capillary wedge pressure or PCWP.
 PRESSURE MEASUREMENTS
PRESSURE MEASUREMENTS
The most important cardiac pressure is that of the left ventricle., because it is a major determinant of systemic perfusion. The pressure in the left ventricle just before systole is called the
left ventricular end-diastolic pressure or LVEDP. This pressure reflects the compliance of the left ventricle - it's ability to receive blood from the left atrium during diastole. When the left
ventricular compliance decreases, the LVEDP rises. MI and left ventricular failure are two examples of when left ventricular compliance decreases.
 CORRELATION OF PRESSURES
CORRELATION OF PRESSURES
There is a close correlation between LVEDP and other cardiac pressures. In the presence of a normal mitral valve, LVEDP is reflected by left atrial pressure or LAP. In the person with a normal mitral valve and normal lung function, the LVEDP is also reflected by the pressure in the pulmonary capillary
bed,pulmonary capillary wedge pressure or PCWP) and the pressure in the pulmonary artery at the end of diastole. This latter pressure is sometimes referred to as the pulmonary artery end diastolic pressure or PAEDP.
 Remember, this concept only hold true for patients with a normal mitral valve and no pulmonary disease.
Remember, this concept only hold true for patients with a normal mitral valve and no pulmonary disease.
Left arterial pressure can be monitored at bedside, but a LAP line can be dangerous because it provides a direct path for air or clots to enter the left ventricle and become systemic emboli. The pulmonary capillary and pulmonary arterial pressures can be monitored at the bedside with a balloon-tipped
catheter placed in the pulmonary artery. With the balloon deflated, one can measure pulmonary artery systolic, diastolic, and mean pressures with the catheter. When the balloon is inflated, it wedges the catheter in a small distal branch
of the pulmonary artery. The pressure recorded is that reflected back from the left atrium through the pulmonary capillary bed. This pressure is the
Pulmonary capillary wedge pressure or PCWP.
Note..these can vary somewhat from institution to institution..
-right atrium mean 0-8 mm Hg; A wave: 2-10 mm Hg; V wave: 2-10 mm Hg
-right ventricle systolic 15-30 mm Hg; end diastolic: 0-8 mmHg
-pulmonary artery systolic 15-30 mm Hg; end diastolic: 3-12 mm Hg
-wedge A wave 3-15 mm Hg; V wave: 3-12 mm Hg; mean: 5-12 mm Hg
-AVO2 difference (mL/L) 30-50
-cardiac output (L/minute) 4.0-6.5 (varies with patient size)
-cardiac index (L/minute/m2) 2.6-4.6
-pulmonary vascular resistance (dynes - second - cm-2) 20-130
-systemic vascular resistance (dynes - second - cm-2) 700-1600
Pressure tracings may be virtually diagnostic of certain conditions.
-Mitral stenosis is associated with a pressure gradient in diastole across the mitral valve (wedge or left atrial pressure vs left ventricular pressure). A large V wave in the pulmonary artery wedge tracing may be seen with mitral regurgitation, since the amplitude of the V wave is affected by left atrial filling from the pulmonary veins as well as the regurgitant volume from the left ventricle.
-Decreases in right atrial pressure, pulmonary capillary wedge pressure, and cardiac index/output can indicate hypovolemia.
-In cases of elevated right atrial pressures with low wedge pressures and low cardiac index/output (especially in the face of an inferior wall myocardial infarction) one may suspect right ventricular involvement and failure.
-Pulmonary congestion due to left ventricular failure or volume overload will increase the pulmonary artery wedge pressure (ie, congestion usually occurs at a wedge pressure in excess of 18 mm Hg and frank pulmonary edema occurs with a wedge pressure in the upper twenties and above).
-Cardiogenic shock and pulmonary edema are characterized by signs of hypoperfusion, with hemodynamic data including systemic hypotension, markedly decreased cardiac index less than 2.1 L/minute/m2, and elevated wedge pressures, often well above 18 mm Hg.
-Septic shock is also characterized by clinical signs of hypoperfusion, but may be differentiated from cardiogenic shock by certain hemodynamic data which often include a normal or near normal wedge pressure, an elevated cardiac index/output, and a marked decrease in systemic vascular resistance.
Pulmonary artery catheters can also be useful in the diagnosis of ventricular septal defects by sampling O2 saturations as the catheter is advanced from the great veins to the right atrium to the right ventricle and out into the pulmonary artery. An oxygen "step-up" from the right atrium to the right ventricle of approximately 10% is indicative of left to right shunting.
In the appropriate setting of acute myocardial infarction and sudden deterioration after a stable course, this diagnosis may be a consideration; right heart catheterization is one method to establish the diagnosis. Other causes of an O2 step-up include coronary fistula draining into the RV, primum atrial septal defects, and pulmonic insufficiency with a patent ductus arteriosus.
Cardiac tamponade is another diagnosis which can be documented by pulmonary artery catheter measurements. Rising intrapericardial pressures interfere with diastolic filling of the heart. Marked increases in the end-diastolic pulmonary artery (PA), right ventricular (RV) , and right atrial (RA) pressures to the same value ("equalization of the pressures") strongly suggest tamponade. Somewhat similar findings may be seen with constrictive and restrictive diseases. Pulmonary hypertension and increased pulmonary vascular resistance can suggest such diagnoses as pulmonary embolism or even mitral stenosis. Care must be taken in the interpretation of all hemodynamic data derived from the catheter.
PLEASE REMEMBER...
1. Compare the values obtained to the patient's normal values rather than an arbitrary standard. If the patient has undergone cardiac Cath within the past few months, pressures obtained at that time may be used as baselines. If not, you must predict general values on the basis of your knowledge of the so-called normal values and your patient's pathology. For example, you would expect the patient with a narrowed tricuspid valve to have an elevated CVP. The patient with COPD probably would have both high CVP and PA pressures.
2. Single readings are not as important as the trend of values.
3. Consider the pressures in relation to each other. If one pressure is measured with a manometer and another with a transducer, you may want to convert them to the same scale. To convert millimeters of mercury (Hg) to centimeters of water, multiply by 1.36. Remember that abnormal values are not always due to a primary pathology of the monitored chamber. For example, and elevated CVP in association with normal or low PA pressures suggests that the cause lies between these two sites, that is, with the pulmonary valve, right ventricle, or tricuspid valve.
4. Remember that a normal valve does not necessarily indicate an absence of pathology. For example, a patient may have a normal CVP but be intensely vaso-constricted due to hypovolemia.
Step up to the blackboard please, and meet our patient. He has just had an MI less than one week ago, and now
it appears he is going into heart failure. He is in serious need of our monitoring his hemodynamic status constantly and closely.
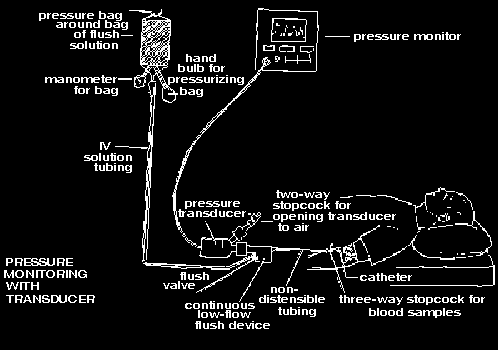
In ordere to do this, we are using a transducer, which is an instrument that converts
pressure waves into electrical energy so they can be displayed on an oscilloscope. We are doing this because his pressure has been too high
for the water manometer. His flush system consists of heparinized 5% Dextrose and he is obtaining this by a countiuous
low-flow flush device. This is most desirable because it is a closed system...and while it has a continuous low flow, if a rapid flow is needed,
you can pull on the "tail" of the device and flush the system without breaking sterility!

 PULMONARY ARTERIAL (PA) LINES
PULMONARY ARTERIAL (PA) LINES
Pumping by the heart results in the development of pressure in the aorta and the arteries. If pressure in the aorta is recorded over time a pressure wave can be observed:
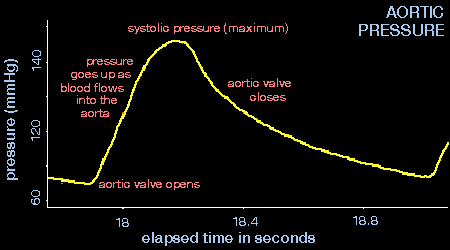
Many factors influence the aortic pressure waveform. Consider the following example:
Greater ventricular filling (more filling time) resulted in greater systolic pressure. Can you think of a basic law of the heart which this situation reflects?
How about the Frank/Starling mechanism. Starling stated that "the energy of contraction is a function of the length of the muscle fibre." So the greater the filling of the ventricles the stronger the subsequent systolic contraction.
Other factors such as aortic valve condition, compliance (elasticity) of the aorta (related to age and disease), vascular resistance, cardiac output and technical considerations of recording can affect the arterial pressure waveform.
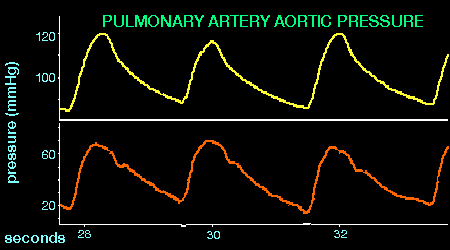
The pulmonary artery pressure waveform is similar in form to, but generally of lesser magnitude than, the aortic pressure waveform.
The type of catheter that allows you to monitor these waveforms is generally referred to by the name of one specific brand of catheter, the Swan-Ganz pulmonary artery catheter.
 Insertion of the Swan-Ganz Catheter
Insertion of the Swan-Ganz Catheter
Contraindications
-severe, uncorrectable coagulopathy
-presence of a left bundle branch block (LBBB) on EKG;
-placement of a right heart catheter may lead to complete heart block (A-V dissociation) if an underlying LBBB is present -local infection at the skin insertion site
-severe hypothermia; in this situation the myocardium is highly irritable and prone to malignant arrhythmias induced by the catheter
-inadequate monitoring equipment; continuous EKG monitoring with blood pressure measurements is necessary during catheter insertion
-patient refusal
Patient Preparation
Technique and risks of the procedure are explained to the patient. When patient is comatose or disoriented, the appropriate guardians should be contacted. Catheterization may be safely performed in an intensive care unit, specialized procedure room with telemetry and fluoroscopy, or a formal Cardiac Catheterization Laboratory. A standard emergency room or regular nursing floor is generally not equipped for this procedure. No specific patient preparation is required and often this procedure is performed on an urgent basis. Whenever possible, aspirin and nonsteroidal anti-inflammatory agents should be discontinued in advance, but this is not absolutely necessary. Effects of heparin or warfarin, however, should be reversed prior to catheterization. If an underlying coagulopathy is suspected (eg, disseminated intravascular coagulation, thrombocytopenia), appropriate laboratory studies should be obtained immediately including a platelet count and PT/PTT. In most cases parenteral sedation is unnecessary; however the use of agents such as meperidine (Demerol) is at the physician's discretion.
Complications
-balloon rupture
-conduction disturbance (ie, new right bundle branch block 5%)
-arrhythmias (3% ventricular tachycardia, 2% ventricular fibrillation)
-pulmonary infarction/pulmonary hemorrhage perforation or rupture of the pulmonary artery
-knotting of the catheter
-thrombosis of a blood vessel (ie, 1% to 2% superior vena cava syndrome)
-pulmonary emboli
-infection (0% to 5%)
-blood loss, including hemothorax, retroperitoneal bleed, etc
-inadvertent arterial puncture (6% femoral)
-pneumothorax and tension pneumothorax (0% to 6%)
-valvular trauma
-disconnection of the introducer apparatus with disappearance into the vein
Equipment
-I.V. pole and pressure monitor manifold, pressure monitor
-normal saline (250-500 mL) with heparin (1000 units) for flush
-pressure bag
-pressure tubing
-stopcocks (3-way)
-cutdown tray (for peripheral approach)
-vein introducer kit
-Swan-Ganz catheter kit
-1% lidocaine for local anesthesia
-bowl of sterile saline (flush and balloon integrity check)
-suture
-instrument set
-3 and 5 mL syringes
-25-gauge needle for anesthesia
-gloves, gowns, masks
-sterile dressing kit (surgical drapes)
-bedside table on which to place instruments
-telemetry monitor for heart rate and rhythm automatic blood
-pressure cuff, A-line
-Betadine scrub
Technique
Swan-Ganz catheterization can take place via a variety of approaches including internal jugular vein, subclavian vein, femoral vein, or brachial vein. The last of these approaches most commonly entails direct visualization of the brachial vein from a cut-down exposure. The procedure should be performed in a closely monitored setting, enabling constant recording of heart rate, rhythm, and frequent blood pressure readings, usually an intensive care unit. The procedure may be performed at the patient's bedside with or without the assistance of fluoroscopic guidance. Sterile technique is required for catheter insertion. The skin at the site of approach is most commonly prepped with a Betadine scrub. Often, if the internal jugular or subclavian veins are utilized, the patient is placed in a Trendelenburg position to assist with central venous distension and ease of access. The physician should scrub and wear gown, mask, and gloves. The patient is then draped with sterile sheets (most institutions drape the patient from head to toe, while others require a sterile field only at the site of access). The patient should be cooperative for catheter insertion. If a patient is uncooperative or becomes uncooperative during the procedure, sedation may be given at the discretion of the physician. Upon initiation of the procedure, the skin and subcutaneous tissue is infiltrated with lidocaine (1%) and a small gauge needle. Deeper tissues may then be infiltrated with lidocaine for the comfort of the patient. A thin gauged needle (21-gauge, 112") is usually attached to a 5 mL syringe and used to localize the vessel of interest for a central venous approach. Once the vessel has been located, a large gauge needle (16- or 18-gauge) is then attached to a syringe and placed into the vessel following the course of the "finder needle." When blood is aspirated easily into the syringe, the syringe is disconnected from the needle and a flexible guidewire is threaded through the needle into the vein. Wire placement can cause a variety of complications, most often ventricular ectopy. If an increase in ectopy is observed, the guidewire should be withdrawn several centimeters. Once the guidewire has been passed into the vessel, the needle is removed from the patient. At no time should the physician lose control of the tip of the guidewire. Failure to control the guidewire can cause serious complications and death if lost in the patient. Once the needle is removed, a dilator is advanced over the guidewire and through the skin, to facilitate passage of a venous introducer. The introducer should be flushed with heparinized saline prior to insertion to avoid air emboli. Once the tract along the guidewire is dilated, the dilator should be slipped off the guidewire (maintaining guidewire position in the vein). The introducer and dilator can then be put together as a unit (dilator within introducer) and slid over the guidewire into the vein, again taking care to control the tip of the guidewire outside the patient's body. After the placement of the introducer and guidewire assembly, the guidewire and dilator should be removed from the patient. This leaves only the venous introducer sheath within the patient. At this point, if the introducer has a side port lumen, venous blood should be aspirated and the introducer then flushed. If blood cannot be aspirated via a side-port lumen, the introducer is incorrectly placed and must be reinserted. No blood should come from the center of the introducer since this piece is usually accompanied by a one-way ball valve which does not allow blood leakage. The introducer should then be secured to the patient's skin with sutures. When the venous introducer has been placed, the Swan-Ganz catheter can then be inserted. Prior to catheter insertion, the balloon tip should be checked under sterile water for leaks and the catheter flushed. The catheter should then be connected to the appropriate pressure monitoring lines and flushed again via the pressure tubing to ensure that the catheter is bubble-free and that a column of uninterrupted fluid exists from the tubing through the tip of the catheter. The catheter can then be guided via the introducer, through the central venous system, through the right atrium, right ventricle, pulmonary artery, and into the wedge position. The catheter usually passes smoothly through the circulation, with the aid of the inflated balloon at its tip. The catheter should never be withdrawn with the balloon inflated. Catheter position can be ascertained by pressure wave forms, although fluoroscopy can be quite helpful in guiding the catheter into the wedge position. A chest radiograph is usually obtained after catheter insertion to verify position, as well as to rule out the possibility of pneumothorax if the subclavian or internal jugular approach was utilized.
 ARTERIAL OR A-LINES
ARTERIAL OR A-LINES
Arterial lines are catheters placed in systemic arteries to facilitate recording of continuous
accurate data about blood pressure in a patient who is hemodynamically unstable, and to allow frequent sampling of arterial blood gases without the need for
repeated arterial sticks. Arterial lines are commonly placed percutaneously in the radial, brachial, or femoral arteries.
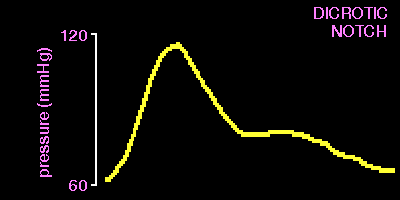
The normal arterial waveform has a sharp upstroke and a more gradual downstroke
with an evident DICROTIC NOTCH, due to a small rise in pressure that occurs at the time of aortic valve closure.
End diastole should be seen very clearly...
 Insertion of The A-Line
Insertion of The A-Line
 Insertion of The A-Line
Insertion of The A-Line
Insertion of an indwelling catheter directly into the arterial circulation for continuous blood pressure (BP) monitoring. Indications
May be divided into three categories:
-hemodynamic monitoring of the unstable patient (acutely hypotensive or hypertensive) including those on vasopressor or vasodilator agents
-multiple sampling of arterial blood, particularly in the mechanically ventilated patient
-determination of cardiac output (less common)
Contraindications
Poor collateral circulation around the artery to be cannulated constitutes a relative contraindication. Thrombus formation at the catheter site is common and can result in distal extremity ischemia if collaterals are inadequate. Also, coagulopathies, systemic anticoagulation (eg, heparin), and interventional thrombolysis are considered contraindications and reversal may be required.
Patient Preparation
The risks and benefits of the procedure are explained. After the site of cannulation is selected by the physician, the area is prepared using povidone-iodine scrub for a minimum of 30 seconds. A sterile technique should be maintained.
Aftercare
Meticulous care is required to avoid line-related infections. Recommendations by the Centers for Disease Control include:
-handwashing prior to any manipulation of the system
-applying topical antiseptics to the insertion site immediately after catheter is placed
-covering the site with sterile dressing
-recording date of catheter insertion and each dressing change -daily inspection of catheter site
-replacing sterile dressing every 48-72 hours with new antibiotic ointment
-flushing of line using normal saline in a closed flush system
-changing flush solution every 24 hours
-changing arterial line site every 4 days or less
-removing catheter promptly at the first sign of infection
Complications
Estimates of significant complications range from 15% to 40%. Thrombosis is the most frequent complication. Incidence of thrombosis increases if:
-the catheter is left in place more that 3-4 days
-a large diameter catheter is used
-multiple puncture attempts are required
-hypotension, decreased cardiac output, atherosclerosis, or hypothermia are present
-prolonged pressure is required to control bleeding after catheter removal; thrombosis rate under optimal conditions is approximately 5% to 8%; symptomatic occlusion requiring surgery is much less <1%)
Infectious complications are also frequent, with the catheter serving as either a primary or secondary site of bacteremia. Factors predisposing to infection include prolonged (more than 4 days) catheter insertion, the use of cutdown for insertion, local inflammation, and infection from a secondary source. Other complications include hemorrhage or hematoma formation, pseudoaneurysms, vasovagal reactions, and local skin necrosis. Distal embolization of small clots or air may occur if improper line-flush technique is used.
Equiptment
Varies somewhat depending on artery selected. A 19- or 20-gauge teflon catheter-over-needle is used in most instances. 16 cm catheters are used for femoral and axillary sites, shorter (114" to 2") catheters are used for radial, brachial, and dorsalis pedis sites. If the Seldinger technique is used, a flexible guidewire is also needed. Other equipment includes sterile gloves, hair covers, povidone-iodine, 1% lidocaine without epinephrine, and 3-0 or 4-0 silk suture and suture equipment.
Technique
The radial artery is generally considered the site of choice; alternate sites include femoral, axillary, brachial and dorsalis pedis arteries. For radial artery cannulation, the presence of collateral flow must first be established using the modified Allen test. Following this, the wrist is dorsiflexed 60 degrees and using a sterile technique 1% lidocaine is used to infiltrate overlying skin. Catheter-over-needle is inserted at a 30 degree angle to skin and advanced until arterial blood is seen in the needle hub. The needle is held fixed while the surrounding catheter is advanced into the artery. The needle is removed and the catheter hub is attached to the connecting tubing. After suturing the catheter in place, a wrist board may be used to stabilize the neutral wrist position. The Seldinger technique may be used for larger arteries. Here, the artery is located with a simple 20-gauge needle. Once arterial blood is returned, a flexible guidewire is passed through the needle; the needle is removed and the teflon catheter is threaded over the guidewire into the artery.
Data Acquired
Graphic waveform of arterial pressure, with pressure on the vertical axis (mm Hg) and time on the horizontal axis
Normal Arterial Pressure Tracing
The peak of each waveform represents the systolic blood pressure and the trough represents the diastolic blood pressure (in mm Hg). Normal values for blood pressure obtained by arterial cannulation are slightly higher than those obtained by routine sphygmomanometry, ranging from 5-20 mm Hg higher. This is due to a combination of physiologic and technical factors, reviewed elsewhere. If indirect pressure readings (ie, cuff pressures) are greater than arterial line readings, instrument error is likely. The entire system (tubing, calibration, seals, catheter, etc) should be carefully inspected; the transmitted arterial waveforms may also appear "damped," further suggesting technical error. A normal "square wave" response is also shown in Figure A. This waveform is seen whenever the tubing system is flushed. Most monitoring systems are equipped with a "flush valve" which can be opened and closed rapidly (routinely performed by nursing staff). A rapid-velocity stream flows through the tubing, removing bubbles and debris. The resulting waveform is by nature artifactual, but abnormalities in its configuration suggest underlying technical problems.

Damped Arterial Pressure Tracing
In normal individuals, peak systolic blood pressures vary somewhat with respiration, a finding difficult to appreciate with bedside sphygmomanometry, but easily observed with direct arterial blood pressure monitoring. When a healthy person inspires, there is a transient fall in blood pressure. On the blood pressure monitoring screen, this appears as a "dip" in the pressure tracings, which returns to baseline during expiration. The maximum drop in systolic blood pressure (pulsus paradoxus) should not exceed 8-10 mm Hg. Values less than this are physiologic and should not be confused with cardiac tamponade.
CRITICAL Values
Cutoff values for hypertension, as defined in textbooks, are the same for blood pressure obtained by arterial cannulation and routine sphygmomanometry. A "hypertensive urgency" is characterized by marked elevations in diastolic (and sometimes systolic) blood pressure, accompanied by retinal hemorrhages, exudates, and papilledema. End-organ damage is likely within several days if blood pressure is not adequately controlled. In a "hypertensive emergency" (malignant hypertension), the retinal findings described are present along with such alarming features as acute renal failure, seizures, blurred vision, mental status deterioration, stroke, and congestive heart failure. End-organ damage is already apparent. Although both hypertensive urgencies and emergencies show marked blood pressure elevations (eg, diastolic blood pressure greater than 120-140 mm Hg), there are no precise cutoff values. These syndromes should not be arbitrarily diagnosed or excluded on the basis of arterial line blood pressure readings alone; they are complex clinical diagnoses. Similarly, no black-and-white cutoff values exist for defining hypotension. Most physicians would consider a systolic blood pressure in the 70-80 mm Hg range abnormal if the individual was previously healthy. However, systolic blood pressures in the 80-90 mm Hg range are not unheard of in the patient with end stage cardiac disease or on multiple vasodilatory agents. Conversely, a "normal" systolic blood pressure of 110 mm Hg may indicate significant hypotension in the dialysis patient whose baseline is 200 mm Hg. A drop in systolic blood pressure during inspiration >10 mm Hg is significant. This increased paradoxical pulse may be seen in cardiac tamponade, severe asthma, pulmonary embolism, and other conditions. Arterial cannulation is useful in monitoring the patient with cardiac tamponade, but is seldom used to make the diagnosis. Disparity in blood pressure readings between direct and indirect measurements greater than 20 mm Hg may occur in shock states. This is due to reflex peripheral vasoconstriction (increased systemic vascular resistance). Korotkoff sounds may be barely audible when direct measurement of central arterial pressures are low-normal. Large discrepancies may also be seen in patients with severe peripheral atherosclerosis (arteriosclerosis obliterans), where systolic pressure drops off dramatically distal to a luminal occlusion. It should be emphasized that inaccuracies may occur in both direct and indirect systems. Clinical importance should be placed on the trends in blood pressure values, regardless of the system used.
Limitations
Accuracy is limited by errors introduced by the equipment, which transforms mechanical energy (pulse) into electrical energy (tracing). Factors such as the natural frequency of the transducer, clamping, and compliance may cause artifact. Other sources of error include improper leveling of equipment, improper assembly, and air in the tubing.
Additional Information
Arterial cannulation is generally considered a procedure of low technical difficulty. The true difficulty lies in avoidance of thrombosis and infection and careful patient selection.
 THE CVP LINE
THE CVP LINE
While most nurses, from med/surg to critical care, have assisted doctors in the insertion of CVP lines, it is important to know the type of doctor you are going to assist with this procedure in order to know how to prepare. Please check out and study the following link for this information, and then return to this page by hitting your BACK key...
VERY IMPORTANT LINK
PLEASE CLICK HERE