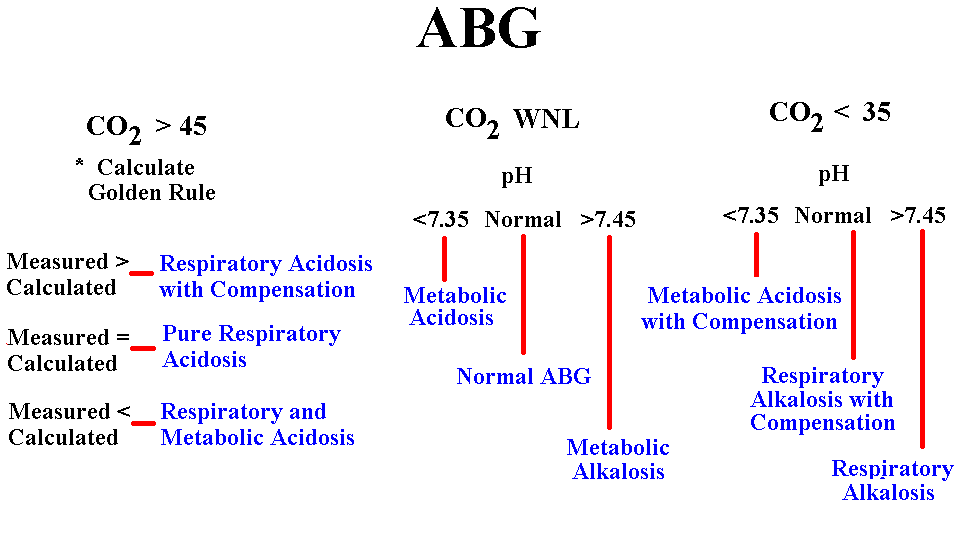
When reading an ABG the first step is to look at the CO2 level in the blood. CO2 or carbon dioxide, is a gas that when mixed with water combines to form carbonic acid (H2CO3). Because we are 60% water by weight any CO2 in the blood can be viewed as acid. We normally need a small amount of this volatile acid to function properly. If the CO2 is greater than 45 you have a Respiratory Acidosis at the very least. The Oxygen content is not used for anything other than to determine hypoxia.
Normal Values: pH 7.4 plus or minus 0.05
CO2 40 plus or minus 5
O2 80 - 100 normal. Less than 80 is hypoxia, greater than 110 indicates pt is on supplemental oxygen.
* The Golden Rule is only calculated in cases of hypercarbia. The Golden Rule states that for every 10 units of CO2 above normal, the pH should inversely change by 0.08 (0.08 is called the conversion factor)
Example: If the patient has an arterial PaCO2 of 58, you would have to establish how many units of 10 it is over normal (Normal is 40). If you subtract 40 from 58 you will have 18 units difference. Remember, however, that you want units of ten. 18 divided by 10 is 1.8 (The 1.8 is the units of ten away from normal)
1.8 times 0.08 = 0.144 (The 0.144 is called the conversion number and represents the amount of change we would expect the addition of these volitile acids should change the pH) Because the increased CO2 inversly affects the pH we need to subtract this conversion from the normal pH (Normal pH being 7.4000)
7.4 minus 0.144 = 7.256 (The 7.256 is the Calculated pH) and needs to be compared to the Actual or Measured pH to complete the interpretation. When you compare these numbers and find the measured pH lower (more acid) then we know there must be other acids dragging the pH down besides the respiratory acid. These acids have to be metabolic acids like lactic and pyruvic acid. If the measured pH is better than the calculated value, there is compensation probably by the renal system. If the calculated and measured pH are fairly close, you have a pure respiratory acidosis.
URL: http://www.monroecc.edu/depts/pstc/parasabg.htm
Updated: February 12, 1998